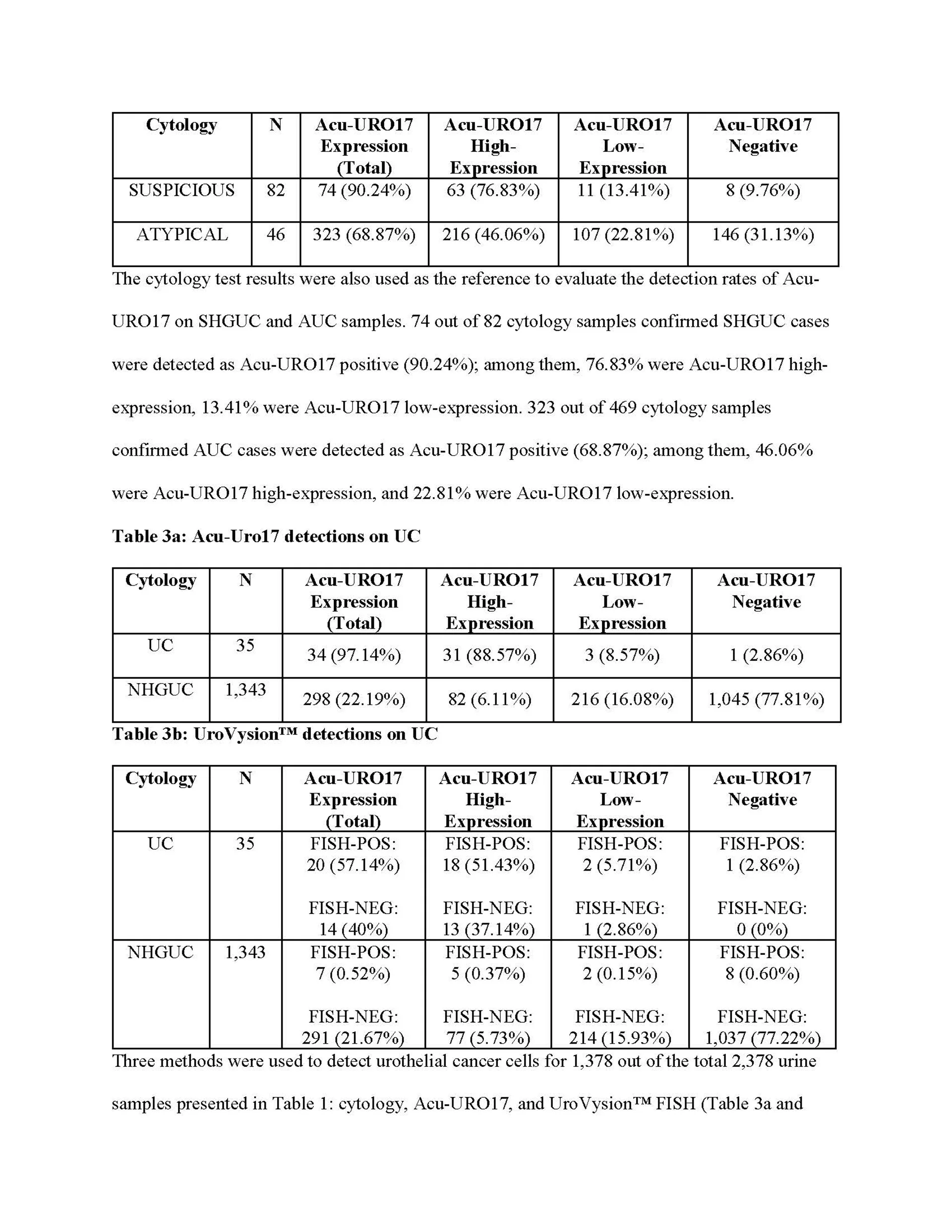
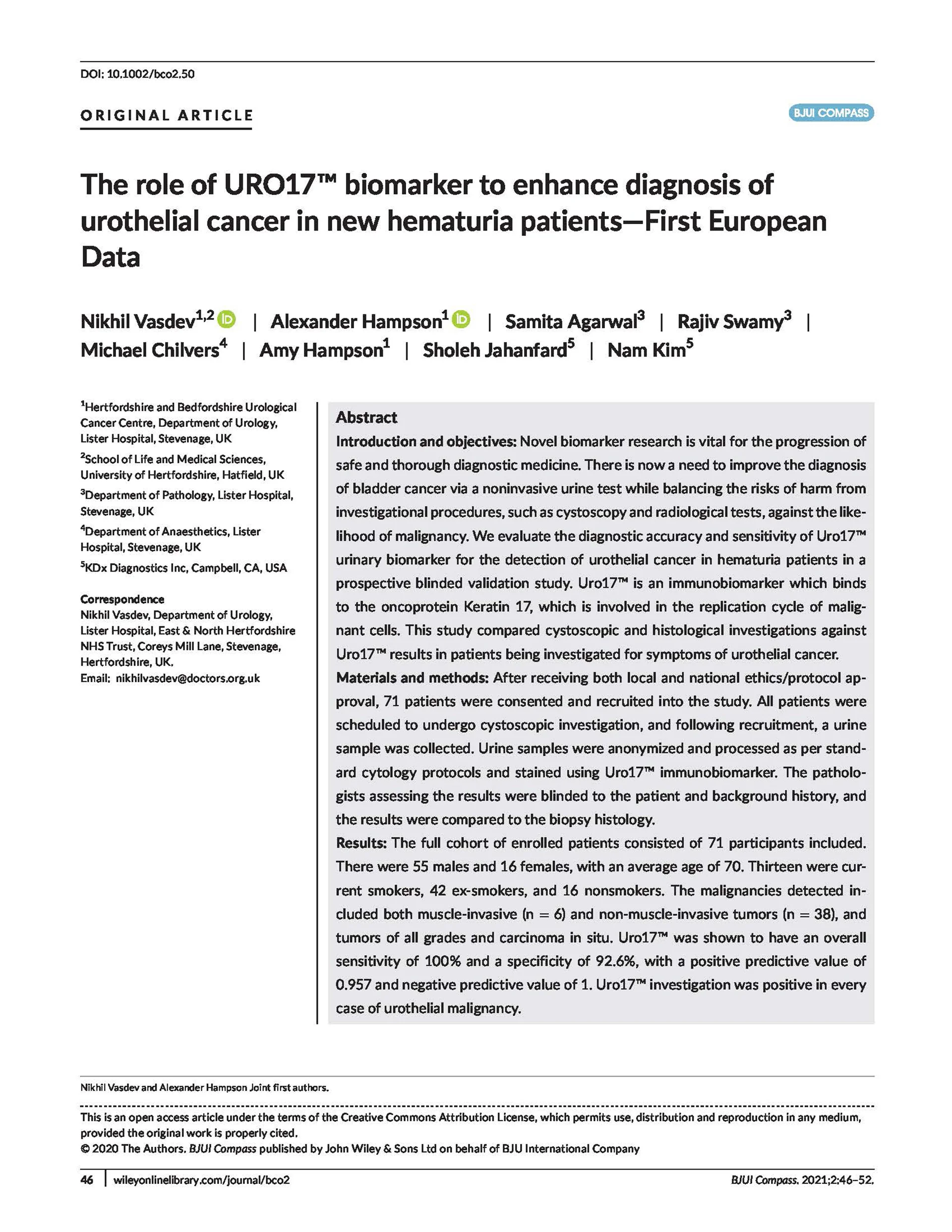
URO17® Bladder Cancer Biomarker
URO17 detects the expression of the protein cytokeratin 17, which occurs prior to the changes required for detection by both cytology and FISH, enabling the assay to detect both low and high-grade disease.
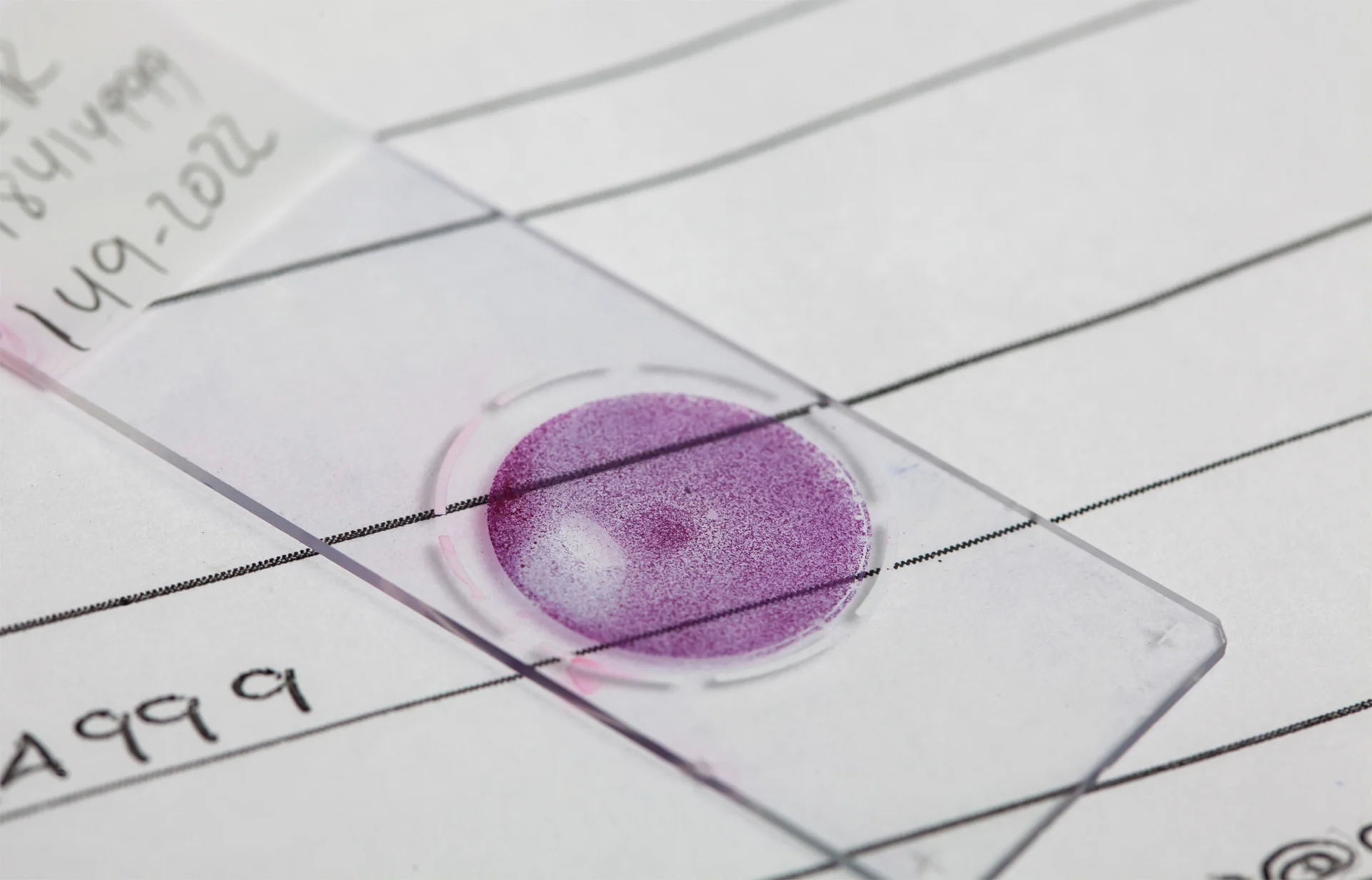
Who Benefits from URO17® testing?
Patients with any of the following:
- Gross or microscopic hematuria
- History of urothelial carcinoma
- History of treatment for urothelial carcinoma
- Atypical/Suspicious cytology result
%
PPV
%
Sensitivity
%
NPV
%
Specificity
Why use URO17®?
URO17 is one of few tests that can identify low-grade bladder cancer. TruCore pathologists can use URO17 as a safeguard between urine cytology and FISH testing so that unneeded testing doesn’t occur.
If URO17 is negative, bladder cancer is not present, saving patients money from running further unnecessary diagnostic testing.

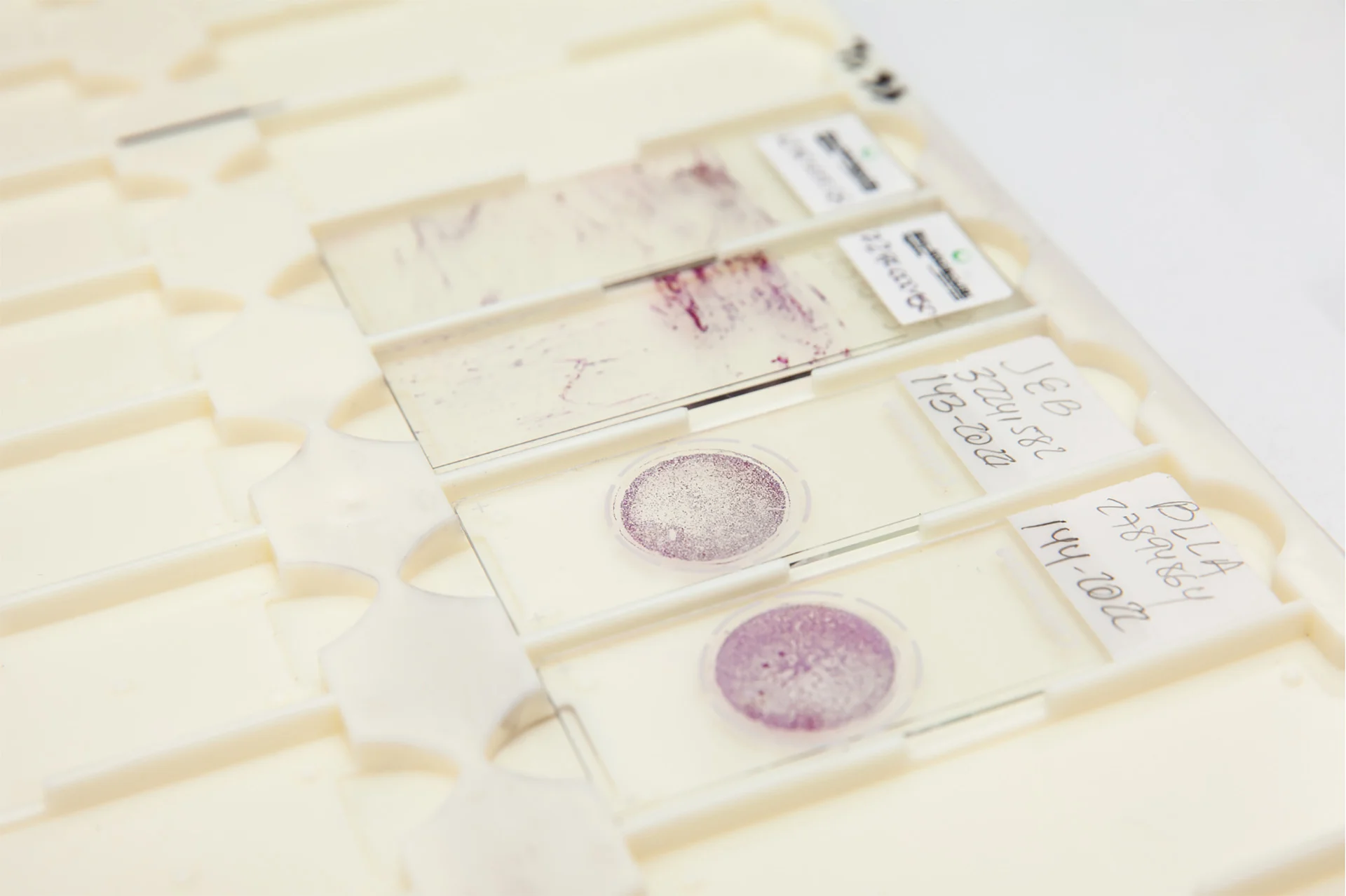
Test Accuracy
Net Present Value (NPV) of 100%
Allows the URO17© test to accurately rule out bladder cancer.
Test sensitivity of >90%
Provides accurate testing with a high ability to identify patients with the related disease.
Positive Predictive Value (PPV) of 91%
Allows the URO17© test to accurately rule in low-grade bladder cancer.
Test specificity of >90%
Provides accurate testing with a high ability to identify patients without the related disease.

Accurate
Cost Effective

Non-Invasive
"Breakthrough Device"
"Before working with TruCore, I would sometimes have questions regarding the reliability of results of more specalized testing such as cytology.
...
I trust the results I receive from TruCore, which minimizes unnecessary cost, inconvenience, and risk to patients."
Jason Greenhalgh, Urologist at Magic Valley Urology
Example Cytology Report with UroVysion and URO17©
Page 1
Page 2